NeuroCytonix, Inc.
Regenerative Technology for Neurological Disorders
Neurological disorders affect millions globally. Since these disorders affect the brain and nervous system of the body, they can have a serious impact on daily life. Finding ways to cope with these disabilities is a major challenge for patients and their families. Through NeuroCytonix, Dr. Trujillo aims to bring hope to people suffering from incurable neurological disorders.
NeuroCytonix, Inc. was founded in 2017 by Dr. Trujillo as a biomedical technology company specializing in regenerative technology for the treatment of neurological disorders. The company was established after years of research and development in neural tissue engineering technology. Its headquarters are in Rockville, Maryland, USA within the Maryland Biotech corridor.
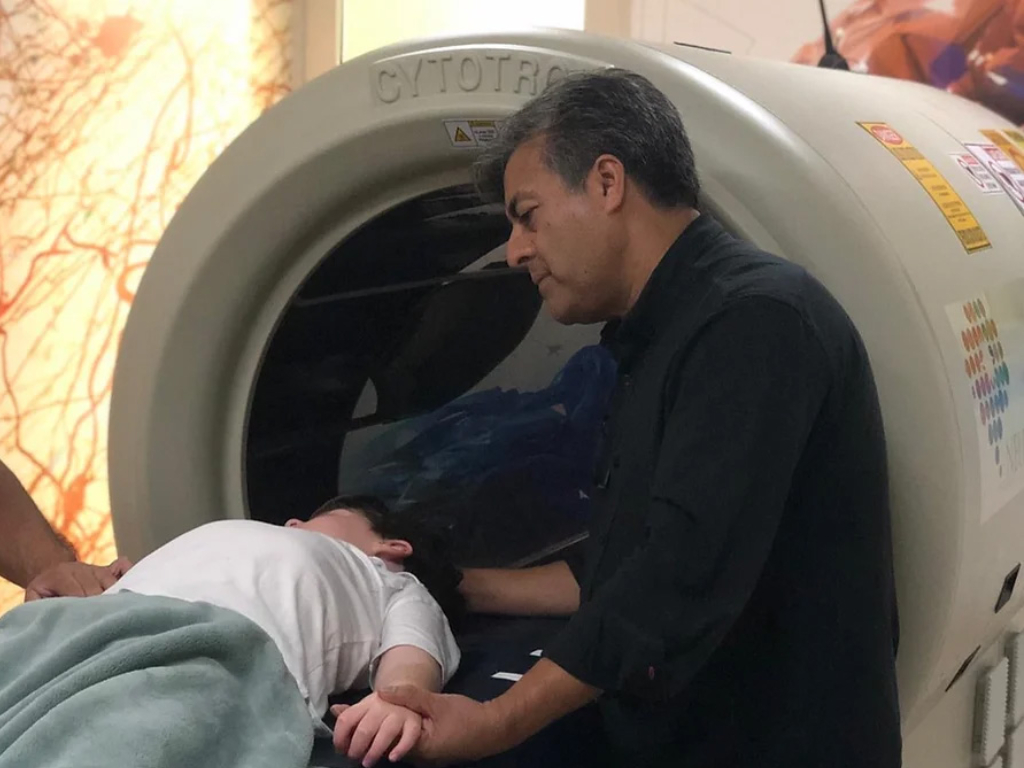
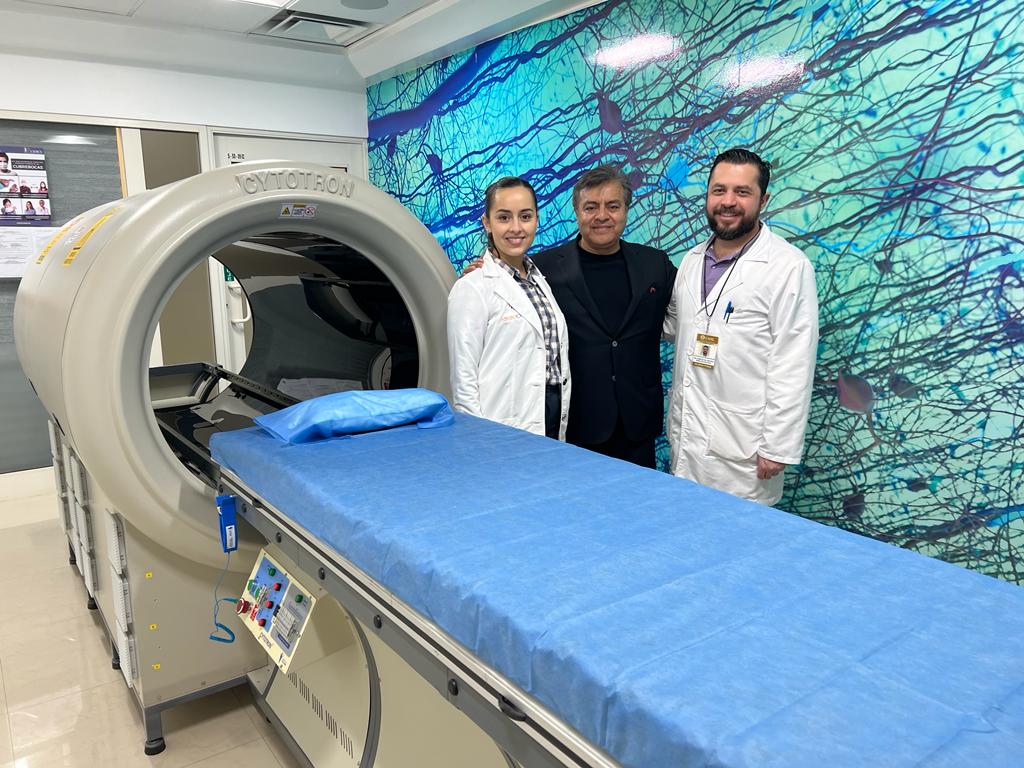
The core of NeuroCytonix technology is the NeuroCytotron , a biomedical device for tissue engineering and regeneration of the nervous system. The NeuroCytotron technology is the invention of Dr. Rajah V. Kumar, a pioneer in biophysics, radiobiology, and regenerative tissue engineering. Dr. Trujillo collaborated with Dr. Kumar to develop NeuroCytotron’s treatment protocols for neurological disorders.
NeuroCytonix is based on the NeuroCytonics program created in 2014 by Dr. Trujillo and Dr. Kumar. The NeuroCytonics program is defined by Dr. Trujillo as a field of bioscience that combines molecular neuroscience, engineering, and quantum physics. The neuronal tissue engineering model relies on the regenerative potential of neuronal progenitor or dormant cells. The protocols were developed after extensive research into several neurological conditions.
The NeuroCytotron reduces symptoms of brain injury-related diseases. It utilizes non-invasive, safe, low-spectrum radio frequencies to generate instantaneous electromagnetic fields to increase the functionality of non-functioning or damaged cells. It is different from traditional approaches to medicine and surgery. The protocols developed include potential treatments for incurable neurological disorders such as cerebral palsy, stroke, autism, and neurological consequences of traumatic brain injury (TBI) and Covid-19.
Clinical trials and research are presently being conducted at NeuroCytonix’s Clinical Research Center in Monterrey, Mexico. Dr. Trujillo and his team completed the first FDA[i]-regulated clinical trial for cerebral palsy evaluating the safety and efficacy of the treatment at this center. The Mexican regulatory authority COFEPRIS [ii] equivalent to FDA has acknowledged the results of the double-blind, randomized, placebo-controlled clinical trial and approved the NeuroCytonix cerebral palsy protocol. Several manuscripts detailing the results of the clinical trial are currently under preparation and will soon be submitted to various scientific journals. In addition to treatment for cerebral palsy in its Mexico center, NeuroCytonix continues to research protocols developed for other neurological disorders.
Know more about NeuroCytonix*The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services.
*COFEPRIS is a member of the International Council of Harmonization (ICH) and Pharmaceutical Inspection Cooperation Scheme (PIC/S) and is a Level IV National Regulatory Authority competent and efficient in the performance of the health regulatory functions recommended by PAHO / WHO.